Kalydeco™ for Sienna?
This is a happy & hopeful post!
I think I've mentioned this before, but when we learned of Sienna's diagnosis, I joined a number of online support groups to help understand CF and the battle ahead. I needed to connect with other parents that knew what I was going through and that I could lean on as a resource. I wanted to know everything there was to know about Cystic Fibrosis.
One group is for individuals on the "miracle drug" Kalydeco™. Some of these individuals take Kalydeco™ because they meet the FDA approved criteria (over age 6 and have the G551D mutation). Others take it "off-label," meaning their doctor's felt as though they would benefit from it and got it approved for them even though they do not have the G551D mutation.
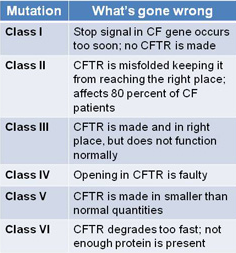
Since learning about Sienna's mutations, I've felt a bit worried about our chances of getting a medicine like Kalydeco™ approved for her. This type of drug aims to correct the underlying defect in the gene; which is unlike the many other drugs available today that just treat the symptoms of Cystic Fibrosis. For this reason, Kalydeco™ and other drugs in trial target a certain gene mutation, or class of mutations. Sienna's mutations are DF508 (class II) and Ex22Dup (not classified). Due to the rarity of Ex22Dup, the mutation (or variant) is not even classified as a disease causing mutation, let alone categorized into one of the six classes below .
I think I've mentioned this before, but when we learned of Sienna's diagnosis, I joined a number of online support groups to help understand CF and the battle ahead. I needed to connect with other parents that knew what I was going through and that I could lean on as a resource. I wanted to know everything there was to know about Cystic Fibrosis.
One group is for individuals on the "miracle drug" Kalydeco™. Some of these individuals take Kalydeco™ because they meet the FDA approved criteria (over age 6 and have the G551D mutation). Others take it "off-label," meaning their doctor's felt as though they would benefit from it and got it approved for them even though they do not have the G551D mutation.
Since learning about Sienna's mutations, I've felt a bit worried about our chances of getting a medicine like Kalydeco™ approved for her. This type of drug aims to correct the underlying defect in the gene; which is unlike the many other drugs available today that just treat the symptoms of Cystic Fibrosis. For this reason, Kalydeco™ and other drugs in trial target a certain gene mutation, or class of mutations. Sienna's mutations are DF508 (class II) and Ex22Dup (not classified). Due to the rarity of Ex22Dup, the mutation (or variant) is not even classified as a disease causing mutation, let alone categorized into one of the six classes below .
*Taken from the CFF.org's website
What has me oh-so-hopeful, is that someone recently posted saying that Kalydeco™ may prove to be effective for anyone with some residual CFTR function. A positive, but low, sweat test result means there is some residual CFTR function. Both of Sienna's sweat tests were in the 70's, which means she may actually benefit from Kalydeco™! (If I haven't already said this, a result over 60 generally means a CF diagnosis. However, many people actually have sweat test results into the 100s).
Reading this had me over the moon. It gave me hope.
For now it's just a question for our clinic team. I don't believe anyone under the age of two is able to use Kalydeco™ (even off label), so getting her on this (if we were to get it approved) is not in the near future. Who knows, by the time she turns two, there may even be a number of other options for her!



Comments
Post a Comment